32 Evidence-based practice and research
The ANA’s Standard of Professional Practice called “Scholarly Inquiry” contains competencies related to evidence-based practice and research. The ANA Standards of Professional Practice are “authoritative statements of the actions and behaviors that all registered nurses, regardless of role, population, specialty, and setting are expected to perform competently.” See the following box for a list of competencies included in the ANA’s Scholarly Inquiry Standard of Professional Practice (American Nurses Association, 2021).
Scholarly Inquiry Competencies in ANA’s Standard of Professional Practice
- Identifies questions in the health care or practice setting that can be answered by scholarly inquiry.
- Uses current evidence-based knowledge, combined with clinical expertise and health care consumer values and preferences, to guide practice in all settings.
- Participates in the formulation of evidence-based practice.
- Uses evidence to expand knowledge, skills, abilities, and judgement; to enhance role performance; and to increase knowledge of professional issues for themselves and others.
- Shares peer-reviewed, evidence-based findings with colleagues to integrate knowledge into nursing practice.
- Incorporates evidence and nursing research when initiating changes and improving quality in nursing practice.
- Articulates the value of research and scholarly inquiry and their application to one’s practice and health care setting.
- Promotes ethical principles of research in practice and the health care setting.
- Reviews nursing research for application in practice and the health care setting (American Nurses Association, 2021).
Reflective questions
- What scholarly inquiry competencies have you already demonstrated during your nursing education?
- What scholarly inquiry competencies are you most interested in performing next?
- What questions do you have about the ANA’s Scholarly Inquiry competencies?
Nursing practice should be based on solid evidence to inform care actions and ensure quality. Evidence-based practice (EBP) is the foundation for providing effective and efficient health care that promotes improved patient outcomes. Evidence-based practice is defined by the American Nurses Association as, “A lifelong problem-solving approach that integrates the best evidence from well-designed research studies and evidence-based theories; clinical expertise and evidence from assessment of the health care consumer’s history and condition, as well as health care resources; and patient, family, group, community, and population preferences and values” (2021). The figure below illustrates evidence-based practice.
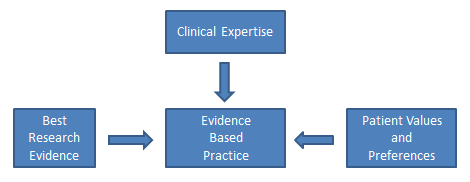
Evidence-based practice is the foundation nurses rely on to ensure their interventions, policies, and procedures are based on data supporting positive patient outcomes. EBP relies on scholarly research that generates new nursing knowledge, along with systematic review of patient outcomes resulting from current nursing practices, to continually promote quality care. EBP encourages health care team members to move new research findings into their practice, referred to as translating evidence into practice. Nurses must recognise the partnership between EBP and research; EBP cannot exist without continual scholarly research, and research requires nurses to evaluate research findings and incorporate them into their practice (Chien, 2019).
Read examples of nursing evidence-based projects from Johns Hopkins.
Quality and Safety Education for Nurses (QSEN) defines evidence-based practice as “integrating best current evidence with clinical expertise and patient/family preferences and values for delivery of optimal health care” (2014). The table below shows the knowledge, skills, and abilities associated with the QSEN competency of evidence-based practice.
| Knowledge | Skills | Attitudes |
|---|---|---|
| Demonstrate knowledge of basic scientific methods and processes. Describe EBP to include the components of research evidence, clinical expertise, and patient/family values |
Participate effectively in appropriate data collection and other research activities. Adhere to Institutional Review Board (IRB) guidelines. Base individualised care plan on patient values, clinical expertise, and evidence. |
Appreciate strengths and weaknesses of scientific bases for practice Value the need for ethical conduct of research and quality improvement Value the concept of EBP as integral to determining best clinical practice. |
| Differentiate clinical opinion from research and evidence summaries. Describe reliable sources for locating evidence reports and clinical practice guidelines. |
Read original research and evidence reports related to area of practice. Locate evidence reports related to clinical practice topics and guidelines. |
Appreciate the importance of regularly reading relevant professional journals. |
| Explain the role of evidence in determining best clinical practice. Describe how the strength and relevance of available evidence influences the choice of interventions in provision of patient-centered care. |
Participate in structuring the work environment to facilitate integration of new evidence into standards of practice. Question rationale for routine approaches to care that result in less-than-desired outcomes or adverse events. |
Value the need for continuous improvement in clinical practice based on new knowledge |
| Discriminate between valid and invalid reasons for modifying evidence-based clinical practice based on clinical expertise or patient/family preferences. | Consult with clinical experts before deciding to deviate from evidence-based protocols. | Acknowledge own limitations in knowledge and clinical expertise before determining when to deviate from evidence-based best practices |
Reflective questions
Read through the knowledge, skills, and attitudes in the table above and work through the reflective questions.
Keeping up-to-date on evidence-based practices
Health care is constantly evolving with new technologies and new evidence-based practices. Nurses must dedicate themselves to being lifelong learners. After graduating from nursing school, it is important to remain up-to-date on evidence-based practices. Many employers subscribe to electronic evidence-based clinical tools that nurses and other health care team members can access at the bedside. Nurses also independently stay up-to-date on current evidence-based practices by reading nursing journals; attending national, state, and local nursing conferences; and completing continuing education courses. See the box below for examples of ways to remain current on evidence-based practices.
Examples of evidence-based clinical supports, nursing journals, and conferences
- American Nurse (published by the ANA)
- American Journal of Nursing
- Journal of Clinical Nursing
- Lippincott Advisor
- UpToDate
- American Nursing Association – Events and Continuing Education
Research
Earlier in this chapter we discussed the process of quality improvement and the manner in which it is used to evaluate current nursing practice by determining where gaps exist and what improvements can be made. Nursing research is a different process than QI. The American Nurses Association (ANA) defines nursing research as, “Systematic inquiry designed to develop knowledge about issues of importance to the nursing professions.” (American Nurses Association, 2021). The purpose of nursing research is to advance nursing practice through the discovery of new information. It is also used to provide scholarly evidence regarding improved patient outcomes resulting from evidence-based nursing interventions.
Nursing research is guided by a systematic, scientific approach. Research consists of reviewing current literature for themes and existing evidence, defining terms and current concepts, defining the population of interest for the research study, developing or identifying tools for collecting data, collecting and analyzing the data, and making recommendations for nursing practice. As you can see, the scholarly process of nursing research is more complex than the Plan, Do, Study, Act process of QI, and typically requires more time and resources to complete (American Association of Colleges of Nursing, 2006).
Nurse researchers often use the PICOT format to organise the overall goals of the research project. The PICOT mnemonic assists nurses in answering the clinical question to be studied (Lansing Community College Library, 2021).
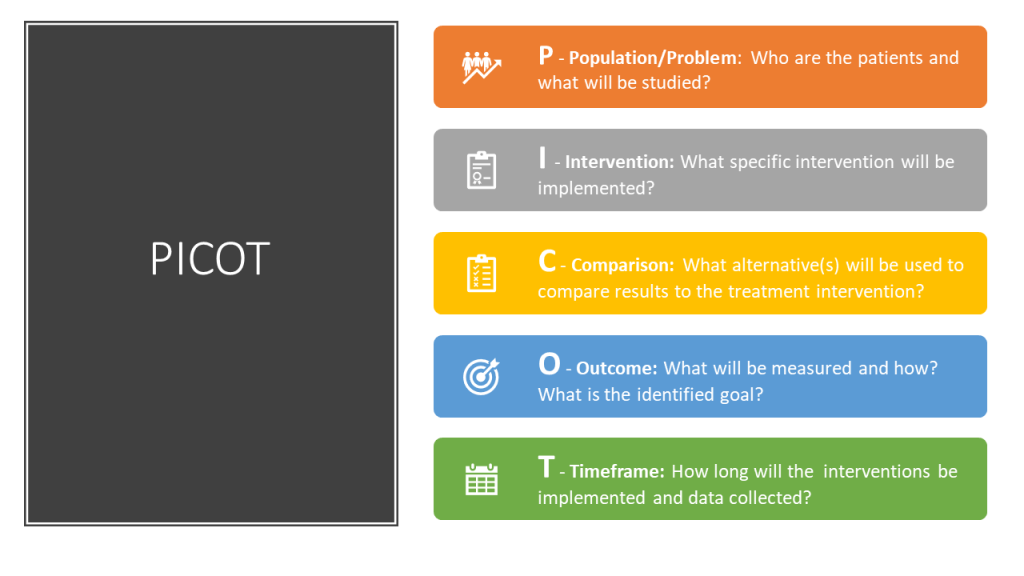
PICOT
P: Population/Problem: Who are the patients that will be studied (age, race, gender, disease, or health status) and what problem is being addressed (mortality, morbidity, compliance, satisfaction, etc.)?
I: Intervention: What is the specific intervention to be implemented with the research population (therapy, education, medication, etc.)?
C: Comparison: What is the alternative intervention that will be used to compare to the treatment intervention (i.e., placebo, no intervention, different medication, etc.)?
O: Outcome: What will be measured and how will it be measured and with what identified goal (i.e., fewer symptoms, increased satisfaction, reduced mortality, etc.)?
T: Time frame: How long will the interventions be implemented and data collected for this research?
After the researcher has completed the PICOT outline, these additional questions should also be considered to protect patients’ rights and reduce the potential for ethical conflicts:
- Was the study approved by the Institutional Review Board (IRB)? The IRB, also known as an independent ethics committee, reviews research studies to protect the rights and welfare of participants (U. S. Food & Drug Administration, 2019). Read more about ethics related to research in Ethical Practice.
- Were the participants protected? Researchers have the responsibility to protect human rights, uphold HIPAA, and respect the personal values of the participants.
- Did the benefits of the intervention outweigh the risk(s)? Researchers have the responsibility to identify if there is a possibility for increased harm to the patients because of the research project.
- Were informed consents obtained? All research participants must provide written informed consent before a study can begin. Researchers must ensure the participants were fully informed of the study, provided risks and benefits, and allowed to exit the study at any time.
- Were vulnerable populations protected? Populations of study that include infants, minorities, children, elderly, socioeconomically disadvantaged, etc., are considered vulnerable populations, and researchers must ensure their rights and safety are accounted for.
After the nurse researcher confirms participants’ rights are protected and has established a PICOT outline, the next step is to design the research study and evaluate sources. Research designs are categorised by the type of data that is collected and reviewed. The three basic types of nursing research are quantitative studies, qualitative studies, and meta-analysis.
- Quantitative: Quantitative studies provide objective data by using number values to explain outcomes. Researchers can use statistical analysis to determine the strength of the findings, as well as identify correlations.
- Qualitative: Qualitative studies provide subjective data, often focusing on the perception or experience of the participants. Data is collected through observations and open-ended questions and is often referred to as experimental data. Data is interpreted by developing themes in participants’ views and observations.
- Meta-analysis: A meta-analysis reviews other independent research studies asking similar research questions. A meta-analysis often collects both quantitative and qualitative data to provide a well-rounded evaluation by providing both objective and subjective outcomes. This research design often requires more time and resources, but it also promotes consistency and reliability through the identification of common themes.
Nurses must understand the types of research designs to accurately understand and apply the research findings. Additionally, only research from peer-reviewed scholarly journals should be used. Scholarly journals use a process called “peer review” to ensure high quality. An article that is peer reviewed has been reviewed independently by at least two other academic experts in the same field as the author(s) to ensure accuracy.
Nurses must also be aware of the difference between primary and secondary sources of scholarly evidence. A primary source is the original study or report of an experiment or clinical problem. The evidence is typically written and published by the individual(s) conducting the research and includes a literature review, description of the research design, statistical analysis of the data, and discussion regarding the implications of the results.
A secondary source is written by an author who gathers existing data provided from research completed by another individual. A secondary source analyses and reports on findings from other research projects and may interpret findings or draw conclusions. In nursing research, secondary sources of evidence are typically published as a systematic review and meta-analysis.
Primary vs secondary sources
By understanding these basic research concepts, nurses can accurately implement current evidence-based practices based on continually evolving nursing research.
References
American Nurses Association. (2021). Nursing: Scope and standards of practice (4th ed.). American Nurses Association.
American Association of Colleges of Nursing. (2006). Nursing research. https://www.aacnnursing.org/News-Information/Position-Statements-White-Papers/Nursing-Research
Chien, L. Y. (2019). Evidence-based practice and nursing research. The Journal of Nursing Research 27(4), e29. https://doi.org/10.1097/jnr.0000000000000346
“Flowchart-sm.png” by Bates98 is licensed under CC BY_SA 4.0
Lansing Community College Library. (2021, August 27). Nursing: PICOT. https://libguides.lcc.edu/c.php?g=167860&p=6198388
“PICOT.png” by Kim Ernstmeyer for Chippewa Valley Technical College, licenced under CC BY 4.0
Quality and Safety Education for Nurses [QSEN]. (2014, October 7). QStudent #4: Evidence based practice. https://www.qsen.org/post/qstudent-4-evidence-based-practice
“Quantitative_methods.png” by Remydiligent1 is licensed under CC BY-SA 4.0
QUT Library. (2020, November 22). Primary vs secondary sources. [Video]. YouTube. https://youtu.be/FZRxYfWYEBI
U. S. Food & Drug Administration. (2019, April 18). Institutional review boards frequently asked questions: Guidance for institutional review boards and clinical investigators. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/institutional-review-boards-frequently-asked-questions
This chapter is adapted from, “Evidence-based practice and research” in “Leadership and management of nursing care” (2022) by Kim Belcik and Open Resources for Nursing, used under a Creative Commons Attribution 4.0 International License, except where otherwise noted and “Chapter 9 – Quality and evidence-based practice” in “Nursing management and professional concepts” (2022), Open Resources for Nursing (Open RN); Ernstmeyer K. Christman E. (Eds.) Eau Claire (WI): Chippewa Valley Technical College, used under a Creative Commons Attribution 4.0 International Licence, except where otherwise noted.

