Introduction to the dental clinic
Consumables
Nicole Stormon
Learning Objectives
Appreciate the broad types of consumables used in dental practice.
Understand the functions of various consumables in dental practice.
Principles
Consumables in dental practice are items that are single use. There are a variety of instruments, products, materials, medicaments and chemicals available. Within these broad categories of consumables the purpose varies dependent upon the procedure and discipline of dentistry.
Instruments and products
Single-use instruments and products are used where reprocessing for reuse may be difficult or not possible. Commonly used dental instruments such as examination and periodontal instruments are often manufactured for single-use. The handles are often made of plastic rather than metals such as stainless steel used in their re-processable counter parts. The shank and working end of single-use instruments are often similar quality to re-processable instruments to maintain the quality and function of the instrument.
Consumable products are used in a majority of dental procedures. Cotton products are generally used for their absorbent qualities for moisture control. Transparent plastic barrier materials can be used to protect instruments and equipment from damage and fluids during use. A large variety of plastic consumable products are available. A plastic product may be required to be disposed after use due to the risk of melting or deformation of the plastic during high temperatures of sterilisation. Some plastic products can be reused, however this is determined by the use of the product and will be discussed further in reprocessing and sterilisation.
How can you tell if it is a consumable or an item that requires reprocessing?
Review the manufacturers instructions. The packaging and instructions will generally tell you if something should be disposed after use or if it can be sterilised or cleaned for reuse. A single use item will always be labelled as single use, sometimes with a symbol of a 2 with a slash or cross through it indicating it cannot be used a second time.
It is common in dental practice to use consumable products to dispense dental materials. Liquid dental materials are dispensed into wells such as dappens dishes and applied using round tipped applicators or brushes. Round tipped applicators are commonly known as microbrushes and come in a variety of sizes.
Various disciplines of dentistry have consumable products specific to the dental procedure. For example, the cup used to remove dental biofilm during a dental prophylaxis are generally consumables and require replacing between patients. During a dental restoration, the practitioner may use a thin metal band that has been manufactured to follow the contours of the tooth and requires disposal after use due to contamination and distortion of the band.
Examples of common consumable products:
Materials and medicaments
Dental materials are used in almost all dental procedures. All disciplines of dentistry use pastes, gels, acids, restorative materials, chemical and medicines. The type and brand of material and medicament selected by the dental professional will vary depending on their training, preference and treatment plan for the individual patient. Many of the materials and medicaments in dental practice are hazardous and require personal protective equipment to handle safely.
Where do I store the material or medicament?
All materials and medicaments will have storage instructions in the material safety sheet. Majority of products will also have the storage temperature written on the container. Always check if your material or medicament has specalised instructions like needing to be stored in the fridge or in a dark location.
Chemicals
Chemicals in dental practice are sometimes used in dental procedures, however a wide range are used in disinfection for infection control purposes. The use of chemical for infection control will be expanded upon in later chapters on infection control and disinfection.
If there are many consumables on the market for purchase, who choses what consumables we have in my dental practice?
The consumables in a dental practice will be determined by what procedures are being undertaken and also operators preference. That is why there is great variability in the stock in dental practices.
Country context
Australia
Materials and medicines for medical purposes in Australia are required to be approved by a regulatory body called the Therapeutic Goods Administration (TGA). The publicly accessible version of the Australian Register of Therapeutic Goods (ARTG) is the reference database of the TGA and provides information on therapeutic goods that can be supplied in Australia. If a therapeutic good is not entered on the ARTG, it cannot be supplied in Australia. There are special circumstances where individuals can access unapproved therapeutic goods.
Under the Workplace Health and Safety Act and Regulations in Australia, Hazardous chemicals must provide a safety data sheet (SDS). Majority of the materials and chemical in dental practice have SDS’s which outline:
- The chemical’s ingredients
- The health and physical hazards
- Handling and storage procedures
- Emergency procedures
- Disposal procedures
Dental practices are required to have copies of the SDS’s for the chemicals they store and use on premise.[1]
The prescription and use of restricted scheduled drugs and medicaments in Australia is regulated by State and Territory legislations. Regulations vary slightly between the States and Territories, however registered Dental Practitioners can generally prescribe and access the full range of scheduled drugs and medicaments relevant to their scope of practice.
|
State or Territory |
Legislation |
|
ACT |
Medicines, Poisons and Therapeutic Goods Regulation 2008 |
|
NSW |
Poisons and Therapeutic Goods Act 1966 |
|
VIC |
Drugs, Poisons and Controlled Substances Act 1981 |
|
SA |
Controlled Substances (Poisons) Regulations 2011 |
|
QLD |
Health (Drugs and Poisons) Regulation 1996 |
|
NT |
Medicines, Poisons and Therapeutic Goods Act 2012 |
|
WA |
Medicines and Poisons Act 2014 |
|
TAS |
Poisons Regulations 2018 |
Practical application to the dental environment
In practice setting, the dental team decide on selection of consumables based on the practice needs as well as availability from the supplier. A decision will be made by the clinician as to which consumable is needed for the procedure. An example in dental practice is a single-use consumable and a reuable instrument for cheek retraction. Both retractors have the same purpose and either may be chosen.
Reusable

Single-use intraoral view
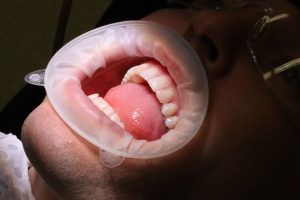
Side by side view. Left: reusable Right: single use

Key Takeaways
- Consumable products can be either single use or reusable. There are several variations and brands available, hence the disparity between practices. It is dependant on the buyer.
- The Therapeutic Goods Administration (TGA) is the regulatory body for medicines used within Australia and dental practice settings.
- Safety data sheets. 2021. Safe Work Australia. The Australian Government. Accessed 01/09/2021: https://www.safeworkaustralia.gov.au/sds ↵

